Blog
Streamlining Pharma Name Clearance: Unified Trademark and Regulatory Review
- Trademark Solutions
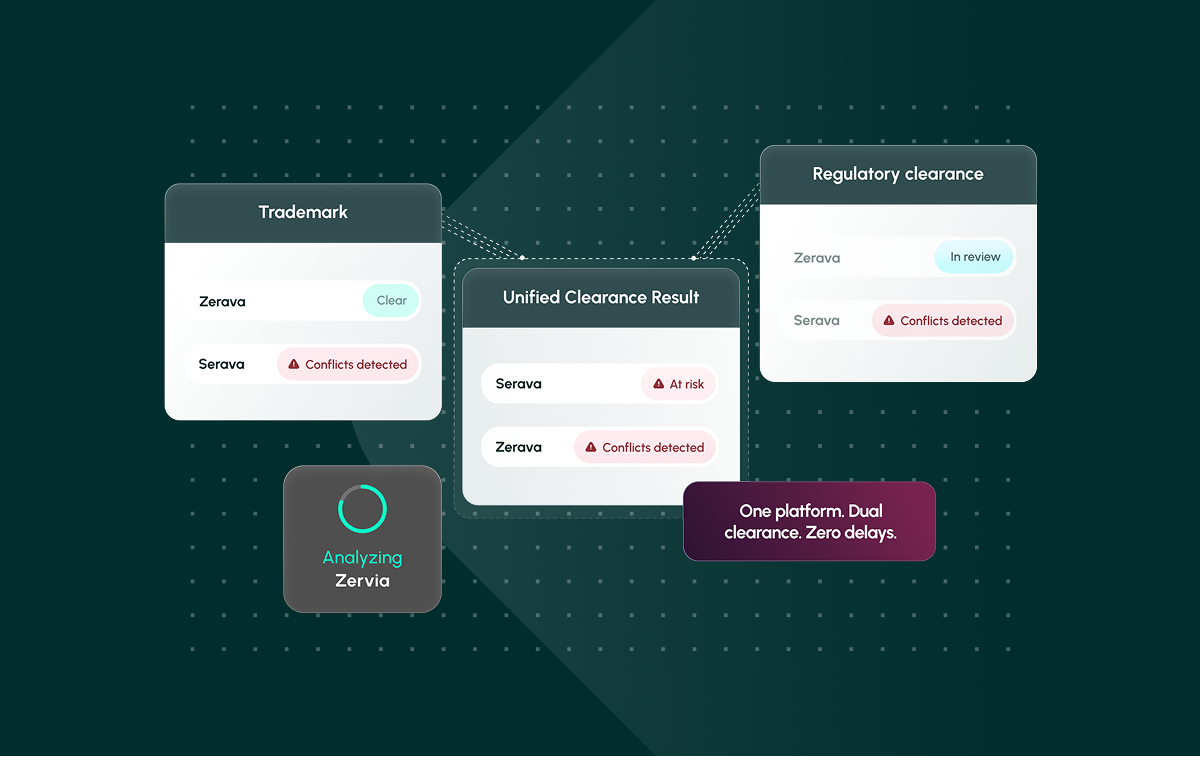
One platform. Dual clearance. Zero delays. Learn how Corsearch Pharma streamlines pharmaceutical name clearance.
Pharmaceutical brand naming has never been easy. With regulators tightening scrutiny and launches growing more competitive, the risk of a name failing late in the process is higher than ever.
This blog explores the dual challenges teams face, why traditional fragmented approaches multiply risk and rework, and how a unified strategy can help companies protect revenue, accelerate time-to-market, and safeguard patient safety.
The dual-hurdle challenge
Pharmaceutical brand naming is not a linear task. It involves two parallel, high-stakes reviews. Legal teams must clear trademarks globally while regulatory teams scrutinize names for patient-safety risks such as look-alike or sound-alike (LASA) confusion. Regulators explicitly evaluate phonetic and orthographic similarity using the FDA’s POCA methodology in the US and comparable criteria in the EU, which means a name can pass legal checks yet still fail on safety.
Treating these as separate, sequential projects multiplies risk and rework. Teams often discover conflicts late in the process, forcing costly rebrands or delaying launches. A better path forward is to unify trademark and regulatory clearance from day one, enabling cross-functional teams to collaborate on the same candidate list and flag high-risk names early. [1]
The cost of fragmentation: duplicated effort, delayed launches, rebrands
Running trademark and regulatory clearance on different systems forces teams to duplicate searches, reconcile data manually, and iterate late when conflicts surface. Those delays are expensive. Independent analyses estimate each day of slippage costs $0.5M–$0.8M in unrealized prescription sales for major therapies, [2] while broader research uses the $1M per day benchmark for launch implications. [3] Small timing slips cascade into material revenue loss and missed market windows.
By combining legal and regulatory screening in one interface, organizations can eliminate duplicated effort, accelerate decision-making, and reduce exposure to both regulatory and trademark risk.
Why “pass legal, fail safety” happens often
Regulatory reviewers actively test for LASA risk using POCA and structured checklists across drug-reference databases such as Drugs@FDA and RxNorm. Names that look or sound like existing products, even if trademark-distinctive, invite rejection to protect patients from medication errors. [4] WHO and regulators consistently flag LASA as a persistent safety threat. [5]
Embedding POCA-aligned scoring and regulatory data integration into the clearance process allows teams to see potential conflicts at the first screening pass. By mirroring the structured similarity checks used by FDA and EMA reviewers, organizations can proactively eliminate non-compliant names, reducing the likelihood of late-stage surprises and costly rework.
Volume and saturation amplify the risk
Most naming programs generate hundreds or even thousands of candidates before a few survive to submission. Without integrated data and automation, teams waste cycles vetting names that will ultimately fail on regulatory similarity or safety signals. Each additional candidate increases the complexity of reviews and the likelihood of missed conflicts. [6]
An AI-driven, high-volume screening approach helps teams focus on the most promising candidates, reducing wasted effort and freeing resources for higher-value creative and strategic decisions.
What “unified” looks like and why it wins
A modern, AI-enabled platform should bring together all datasets relevant to pharma name clearance: trademark registers, common-law, domain data, and regulator sources including the Orange Book, Drugs@FDA, RxNorm, and EMA guidance.
Regulator-aligned methods are embedded natively, including POCA-style similarity scoring and LASA heuristics, so potential conflicts are flagged at the first screening pass. AI-driven triage clusters look-alike and sound-alike candidates, prioritizing by risk to streamline reviews and reduce manual reconciliation.
Finally, results should be standardized into a single, exportable report that legal, regulatory, and marketing teams can trust. Collaboration, annotation, and audit-ready evidence all help organizations move from “hope it clears” to confidence at speed, a capability delivered by Corsearch Pharma, which brings trademark and regulatory clearance together in a single, integrated platform.
Business outcomes you can quantify
By using Corsearch Pharma, legal, regulatory, and marketing teams can achieve measurable improvements in clearance efficiency, risk reduction, and cost savings.
Fewer late rejections by resolving legal and regulatory conflicts together, reducing “back to creative” loops that trigger rebrand costs and slide timelines.
Faster time-to-decision by removing duplicate searches and manual reconciliation. Every day saved protects revenue (recent ranges: $0.5M–$0.8M per day in unrealized prescription sales). [2]
Lower total cost of naming by screening out non-starters earlier and reducing rounds of external review and litigation risk post-launch.
How to operationalize a unified approach this quarter
Teams can implement a unified, efficient workflow from day one with Corsearch Pharma, ensuring that trademark and regulatory clearance are aligned and actionable.
Start unified at intake: Run trademark conflicts and POCA-aligned similarity checks on the same candidate list and rank by composite risk.
Gate with shared criteria: Align legal and regulatory thresholds to FDA best practices and EMA guidance so cross-functional teams triage on identical evidence. [4]
Instrument the loop: Track cycle times from ideation to shortlist. Quantify days saved and rejected names avoided to demonstrate ROI to leadership. Use time saved multiplied by daily revenue at risk to model impact. [2]
Making pharmaceutical name clearance faster and safer
Pharma naming is not just a trademark problem or just a regulatory problem. It is both. Fragmented processes guarantee rework. Corsearch Pharma is the first industry-specific solution to unify trademark and regulatory clearance in one platform, enabling legal, regulatory, and marketing teams to protect revenue, patient safety, and creative investment while accelerating launches.
With flexible POCA scoring, integrated regulatory data, AI-assisted screening, and collaboration features tailored to regulated environments, teams can move from “hope it clears” to confidence at speed.
Let’s talk
Find out what Corsearch Pharma can do for you. Talk to one of our experts and see a demo tailored to your pharma team’s needs.
References
[1] U.S. Food and Drug Administration, Best Practices in Developing Proprietary Names for Human Prescription Drug Products, 2020, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/best-practices-developing-proprietary-names-human-prescription-drug-products-guidance-industry
[2] Applied Clinical Trials Online, How Much Does a Day of Delay in a Clinical Trial Really Cost?, Tufts CSDD, 2024, https://www.appliedclinicaltrialsonline.com/view/how-much-does-a-day-of-delay-in-a-clinical-trial-really-cost-
[3] The University of Texas at Austin, Benchmarking the Cost of Delayed Drug Launches, 2023, https://repositories.lib.utexas.edu/bitstreams/5306e329-39d9-4af3-8b37-9f46000e3112/download
[4] U.S. Food and Drug Administration, Phonetic and Orthographic Computer Analysis (POCA) Program, 2024, https://www.fda.gov/drugs/resources-drugs/phonetic-and-orthographic-computer-analysis-poca-program
[5] World Health Organization, Medication Safety for Look-Alike, Sound-Alike Medicines, 2023, https://www.who.int/publications/i/item/9789240058897
[6] U.S. Food and Drug Administration / European Medicines Agency, Guidance on Aligning with Official Name-Safety Criteria Throughout Development, 2024, https://www.fda.gov/drugs/medication-errors-related-cder-regulated-drug-products/update-phonetic-and-orthographic-computer-analysis-tool