Blog
GLP-1s: The Good, The Bad, The Ugly
- Brand Protection

In a time when discussion on pharmaceutical products is limited mostly to those interested in the field, one topic has continuously made headlines for the past couple of years: GLP-1 receptor agonists.
Of course most people would know them by their brand names: Ozempic, Mounjaro, Rybelsus, Bydureon and Trulicity among others.
The reason for the popularity (or notoriety) of those medicines is attributed to their off-label use as aesthetic weight loss medications. This issue is so wide-spread that in the US, the use of GLP-1s by non-diabetics has risen by 700% since 2019.
What Are GLP-1s and How Do They Work?
Glucagon-like peptide-1 receptor agonists (GLP-1s) are prescription-only medications that help regulate the glucose levels released in the blood stream, resulting in a reduced appetite.
GLP-1s are used to treat type-2 diabetes and/or obesity alongside a reduced calorie diet and regular exercise. Their effectiveness has resulted in multiple people seeking a prescription so they can use GLP-1s for aesthetic weight loss, which is not an approved use of said medicines.
To make things worse, the unsolicited “endorsement” of such medicines by celebrities and influencers has only added to their popularity and caused a consumer frenzy that has raised demand to unprecedented highs.
To put matters into perspective, data from 2025 suggests that the size of the genuine GLP-1 market was estimated at approximately $63 billion. While some would consider the figure to be jaw-dropping, it could even be a lot higher had it not been for the ongoing drug shortage, which has greatly impacted the GLP-1 market.
The same data supports a growth projection for GLP-1s to more than $250 billion in the next 8 years, with a sharp increase in websites promoting fake GLP-1 products:
Source: McAfee
This is also supported by the fact that there are over 330 companies currently engaging in the development of GLP-1s, spanning from Big Pharma to emerging start-ups, as well as the market debut of the as of yet unauthorised retatrutide, a new GLP-1 Active Pharmaceutical Ingredient (API), developed by Eli Lilly,which has already made waves in weight-loss and has been nicknamed “Triple G”.
While everyone seems to want a piece of this lucrative pie, some are willing to go a step further in order to maximise their share. Unsurprisingly enough, bad actors have flooded the market with counterfeit GLP-1s, taking advantage of the excessively high demand for the products, the ongoing medicine shortage, the recently increased prices of the original products (Eli Lilly announced a price increase for Mounjaro from GBP 122 to GBP 330) and of course, the influencer and celebrity advocacy in favour of GLP-1s.
These optimal conditions have resulted in the culmination of thousands of online offers for counterfeit GLP-1s; unchecked, unauthorised and unapproved stock ready for sale with the promise of “miracle results”.
The Rise of Counterfeit GLP-1s
Without having to undergo years of R&D or any regulatory checks and balances, counterfeit versions of GLP-1s can nowadays be found almost anywhere, from illegal online pharmacies that require no prescription to beauty salons and medical spas.
Carefully curated online ads with impressive before and after pictures, claims that this product is superior to the rest for weight loss, fake endorsements and unauthorised use of official brand or international organisation logos are just a few of the tactics deployed by skillful scammers trying to legitimise their offering and amplify their reach.
Sometimes, it takes the trained eye of an expert to be able to spot this type of fraudulent activity and take action against it before it can spread too wide.Of course, taking down the online content or seizing the products from brick and mortar stores has a real life impact for consumers.
The risks that arise from the use of counterfeit GLP-1s can vary in severity, from ineffective treatments to threatening conditions such as hyperglycemia or cardiovascular issues.
This is due to the fact that counterfeit GLP-1s could include harmful ingredients, lack the Active Pharmaceutical Ingredient (API) that gives a medicine its properties or include it in an incorrect dosage. Moreover, the fact that most GLP-1s come in a syringe, means there could also be severe skin irritation at the point of injection as an additional adverse reaction.
User behaviour over time shows the use of GLP-1 agonists increasing since 2011 to present day:
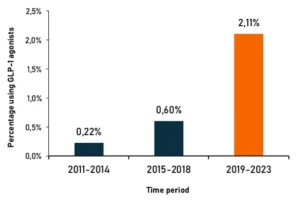
Source: Mabion Biologics
To their credit, Pharma companies have really stepped up to educate and support their patients who are asking “are GLP-1s safe?”, as well as set out to limit the proliferation of fake GLP-1s.
They are regularly publishing educational pieces on their websites on how to spot counterfeit indicators on their products, they have teamed up with regulators like the FDA in the US to expose scammers and they have launched campaigns for further consumer awareness, like the recent #HealthnotHype campaign launched by EMA to spread the word about how to use GLP-1s in a safe and responsible way.
In those efforts, they would need a trusted partner to help them navigate the internet and compliment their efforts by quickly and efficiently removing any harmful online content.
Lastly, in their efforts to safeguard patients, the industry would greatly benefit from entering into a new era of regulatory protection, with an in depth review of the prescription requirements as well as the framework around compounded medicines.
With many confirmed incidents of people reporting fake data about their BMI to acquire a GLP-1 prescription and that data later being processed by an online system rather than an in-person physician, it is easy to understand where change needs to start. In the US, compounding pharmacies have also contributed to the problem, as they are not bound by the same marketing restrictions as prescription medications, and can thus be widely advertised as personalised and cost-effective alternatives to the branded drugs.
It is, therefore, imperative that changes are made that align the needs for a safer and more robust supply of GLP-1s to patients with a modern and effective regulatory framework.
As more and more Pharma companies are putting their hats in the GLP-1 race, with names such as Pfizer, Zealand Pharma and Amgen due to launch their own medications in the next 5 years, it is evident that protecting the integrity of these drugs and their patients is of pivotal importance.
By combining a multi-channel monitoring and enforcement program with a closer collaboration with enforcement agencies and a more robust regulatory framework, there is an obvious need to battle counterfeit GLP-1s and discourage off-label use, while educating people on the harmful side-effects.
After all, there is a lot more to lose than just a few pounds!



